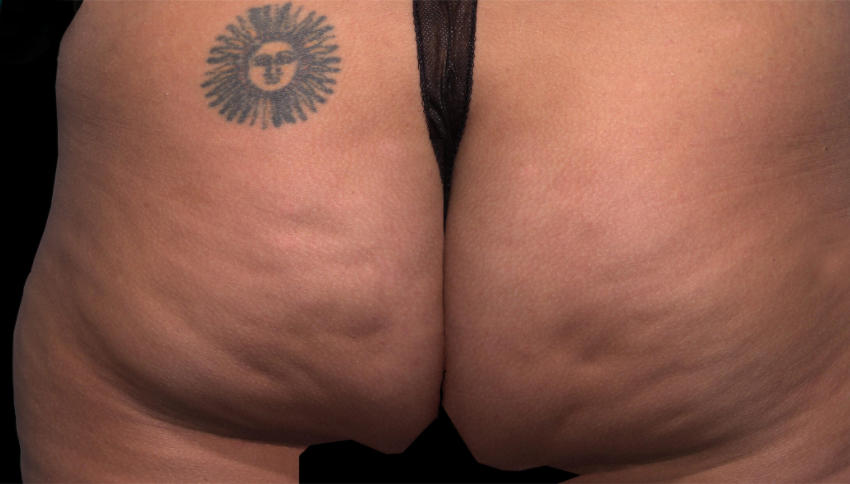
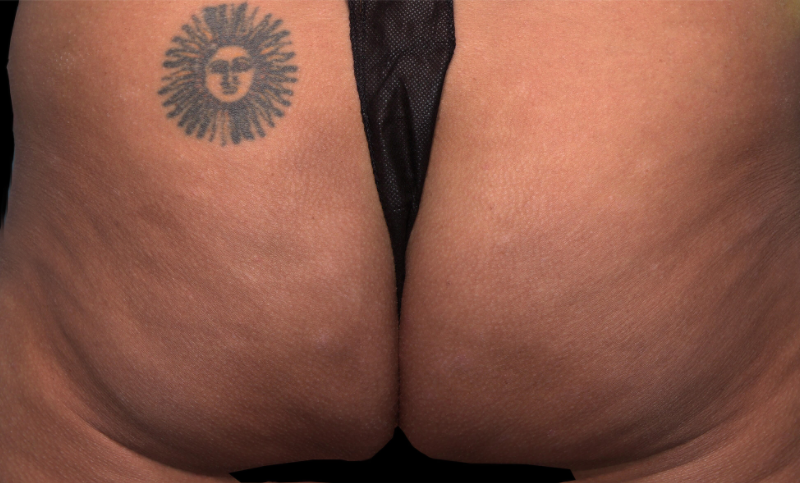
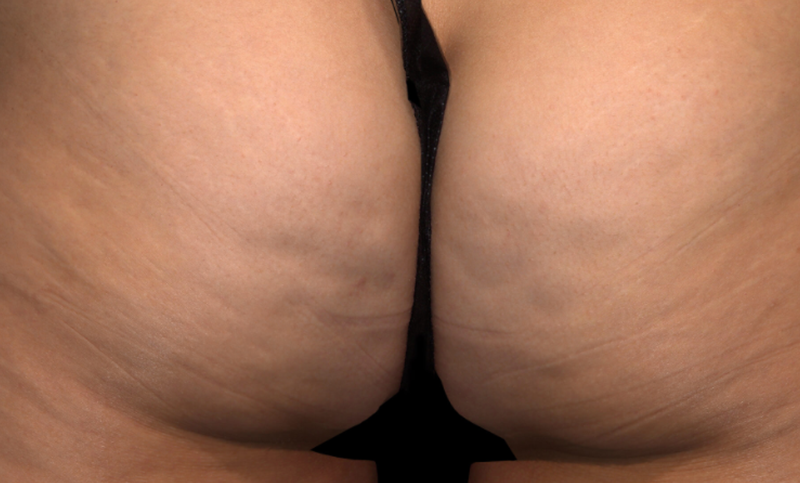
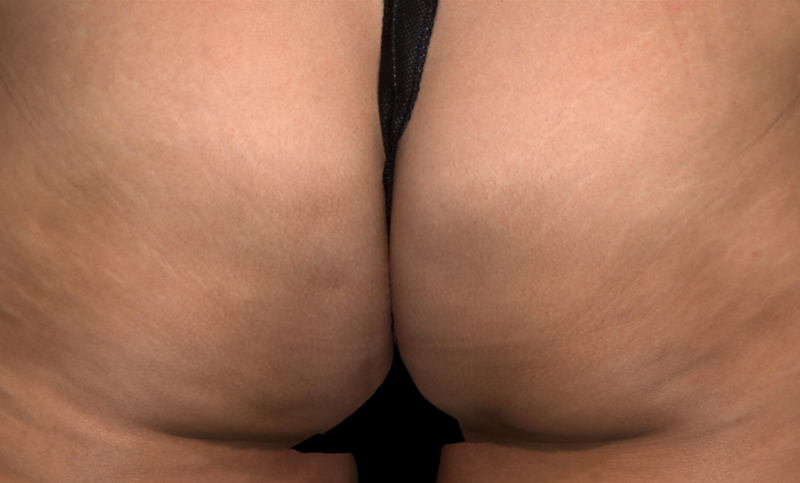
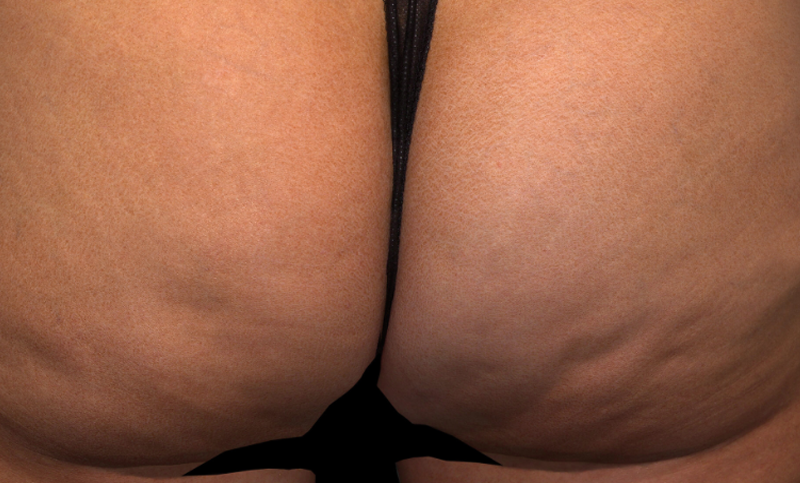
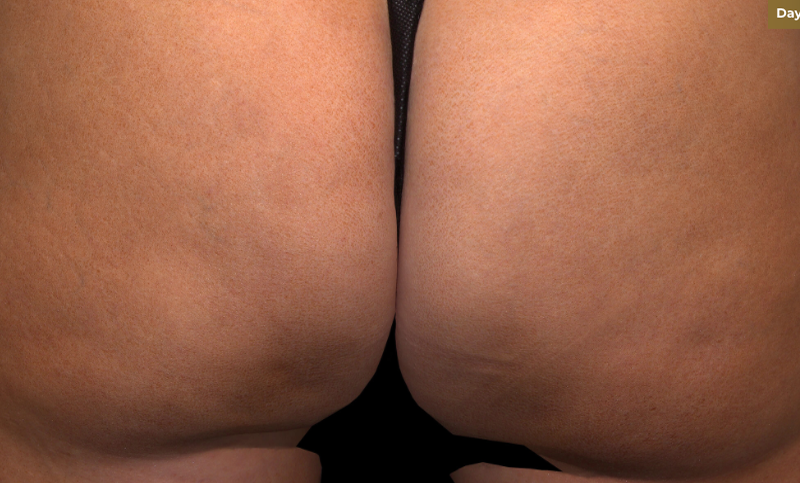
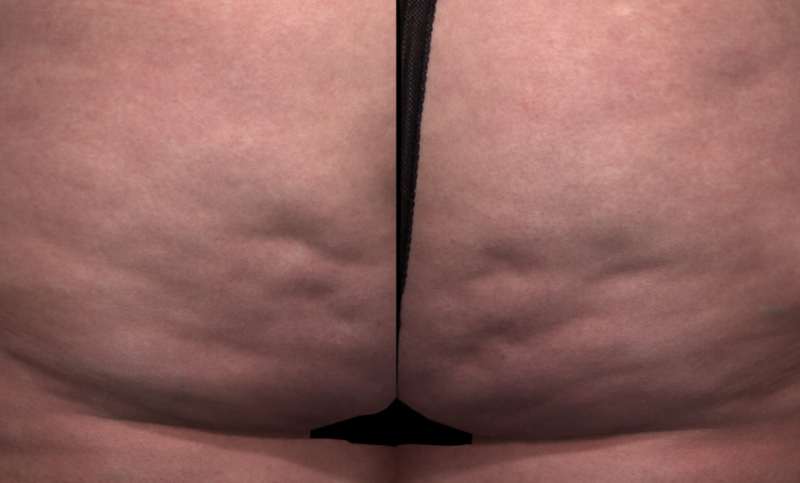
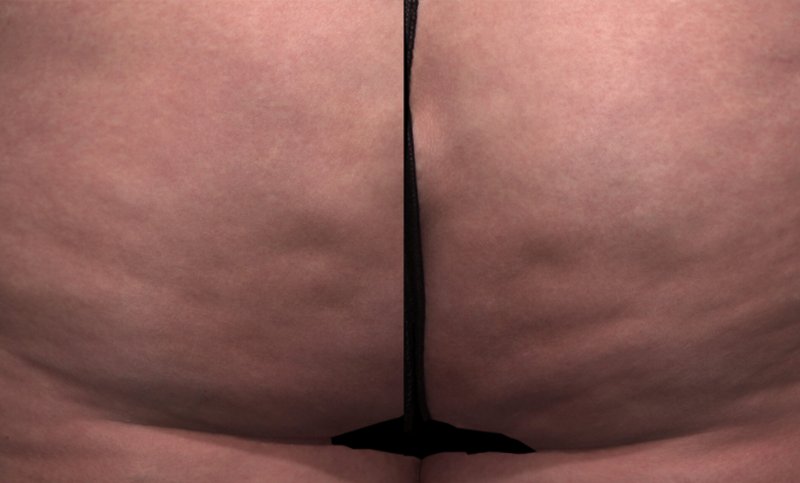
Meet QWO (collagenase clostridium histolyticum-aaes).
QWO is the first and only FDA-approved injectable for cellulite.
Available now at our practice.
QWO is a prescription medicine used to treat moderate to severe cellulite in the buttocks of adult women. Talk to your healthcare provider to see if QWO may be right for you. There are risks associated with this product. You should not receive QWO if you are allergic to any collagenase or the ingredients in QWO, or have an active infection in the treatment area. Serious side effects: allergic (hypersensitivity) reactions, including anaphylaxis, and injection site bruising may occur. These are not all the side effects of QWO.